Background:
Multiple Myeloma (MM) is a rare and heterogeneous blood cancer of the plasma cells. Various new therapies have been introduced and are used in combination to combat the heterogeneity. Despite ongoing research and the development of innovative therapies, improvements for the fast progressing (high-risk) patients have been limited. Disease behavior and clinical outlook of patients that look very similar in the absence of clinical and cytogenetic high-risk features, vary considerably. Their fast progression could not have been foreseen at diagnosis as their initial features were uninformative. In order to identify high-risk patients for selective innovative treatment strategies in trials and clinical practice, it is important to have a conclusive assessment of the patient's risk profile at diagnosis. This assessment should include their Gene Expression Profile (GEP).
Aim:
To show that GEP is an important and indispensable contributor in selecting high-risk MM patients.
Method:
Six datasets were combined: MRC-IX (n=180), HOVON-87/NMSG-18 (n=148), EMN-02/HOVON-95 (n=249), GIMEMA-MMY-3006 (n=112), a Czech cohort (n=27 E-MTAB-1038) and the MMpredict non-trial cohort (n=89, [Chen YT. et al. CLML. 2019 19(10):e60-61]). Pooled, a total of n=805 MM patient samples were available including corresponding GEP data, iFISH, ISS annotation and Overall Survival (OS). Patients were risk classified in three strata as proposed by Kuiper R. et al. Blood. 2015 Oct 22;126(17):1996-2004: Low Risk (LR) as SKY92 standard risk + ISS I; Intermediate Risk (IR) as SKY92 standard risk + ISS II or III; and High Risk (HR) as SKY92 high risk. Subsequently, each stratum is further refined into: patients with high-risk iFISH t(4;14) and/or del(17p) (iFISH); and patients without these markers (non-iFISH). The Cox proportional Hazards model was applied to estimate survival.
Results:
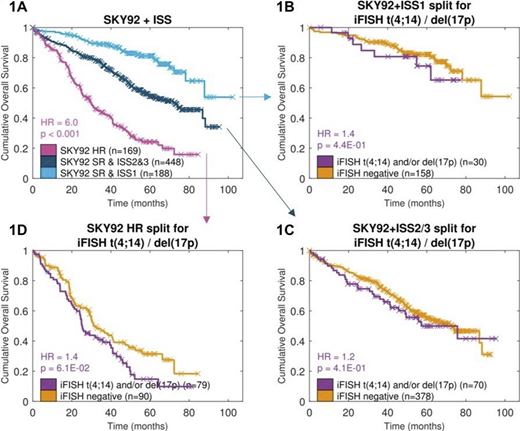
The combination of SKY92+ISS identified three risk strata in the cohort: n=188 for LR (23%), n=448 for IR (56%) and n=169 for HR (21%). The OS of the HR stratum was significantly shorter than the LR stratum (hazard ratio=6.0, p<0.001), see Figure 1A. For all three strata, the comparison of iFISH versus non-iFISH patients resulted in a not significant OS difference: hazard ratio=1.4 and p=0.44 for LR, Figure 1B; hazard ratio=1.2 and p=0.41 for IR, Figure 1C; and hazard ratio=1.4 and p=0.061 for HR, Figure 1D. Our analyses show that for 44% (79 out of 179) of iFISH patients, the traditional high-risk classification is correct. However, GEP identifies another 90 out of 169 (53%) patients with comparable poor outcomes that were missed since they belong to the non-iFISH group, Figure 1D. Lastly, 30 out of 179 (17%) iFISH patients (considered high risk), show a mild course of the disease (median survival not reached at 80 months), Figure 1B. To summarize, without SKY92+ISS, many high-risk and low-risk patients remain unidentified.
Conclusion:
Currently used risk stratification methods are sub-optimal and the use of these markers leave 53% of high-risk patients unidentified. This might impact the clinical strategy for that unidentified high-risk patient as a physician could have escalated their treatment. Furthermore, it might influence the conclusion on the effectiveness of investigational therapeutic treatment in clinical trials. Lastly, clinical trials focused on high-risk patients, will reach full inclusion much faster and obtain earlier read-outs as GEP, here SKY92, identifies additional and true fast progressing high-risk patients. SKY92 should therefore be added as risk stratification method for a holistic view on a patient's course of disease.
Figure 1: Kaplan Meier plots for the three SKY92 + ISS strata: low risk (LR; figure 1B), intermediate risk (IR; figure 1C) and high risk (HR; Figure 1D) and their respective splits based on presence of iFISH markers t(4;14) and/or del(17)p or absence of both.
Van Vliet:SkylineDx: Current Employment, Current equity holder in private company. Chen:SkylineDx: Current Employment, Current equity holder in private company. Van Beers:SkylineDx: Ended employment in the past 24 months. Kuiper:SkylineDx: Current Employment, Current equity holder in private company. Valent:SkylineDx: Current Employment, Current equity holder in private company. Spaan:SkylineDx: Current Employment, Current equity holder in private company. Zamagni:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cavo:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Speakers Bureau; Karyopharm: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Oliva:Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees. Larocca:Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Chng:Abbvie: Honoraria; Novartis: Honoraria; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Waage:Janssen: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy; Shire: Honoraria. Sonneveld:Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Skyline Dx: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal